

图文导读
Abstract
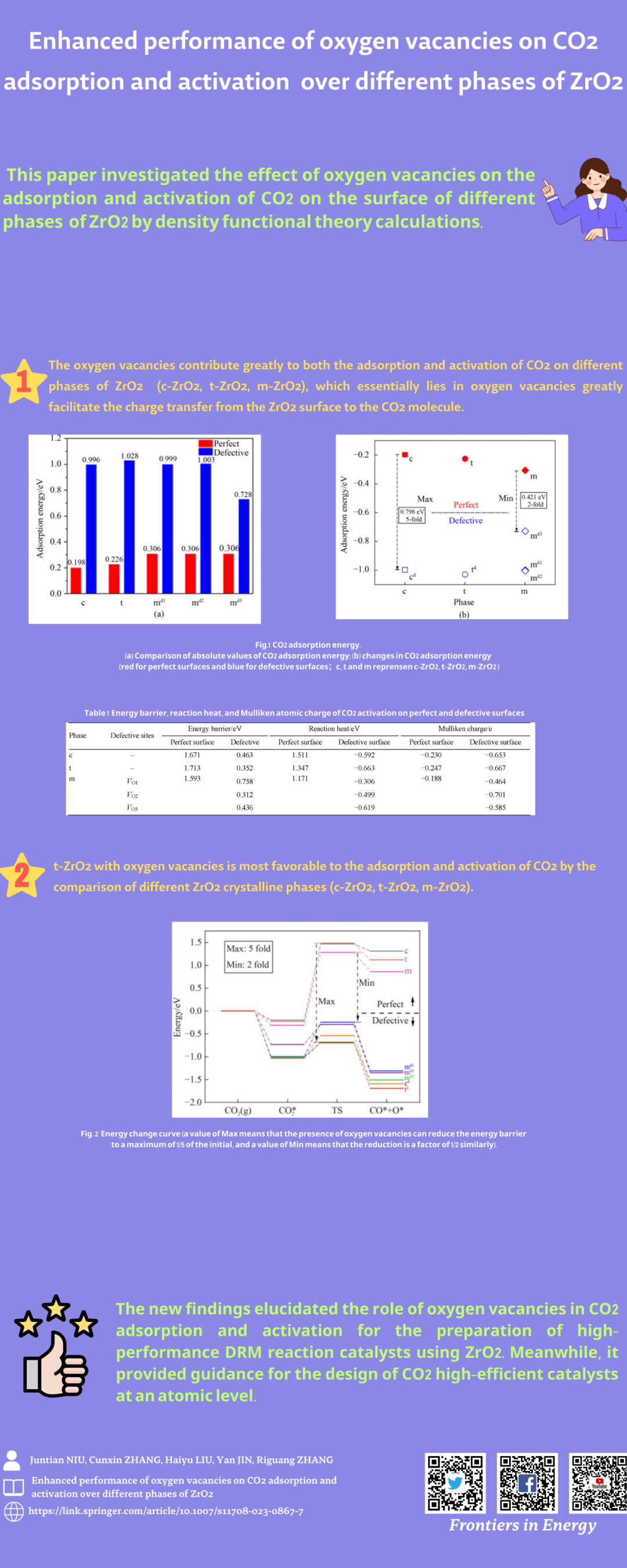
The effect of oxygen vacancies on the adsorption and activation of CO2 on the surface of different phases of ZrO2 is investigated by density functional theory (DFT) calculations. The calculations show that the oxygen vacancies contribute greatly to both the adsorption and activation of CO2. The adsorption energy of CO2 on the c-ZrO2, t-ZrO2 and, m-ZrO2 surfaces is enhanced to 5, 4, and 3 folds with the help of oxygen vacancies, respectively. Moreover, the energy barrier of CO2 dissociation on the defective surfaces of c-ZrO2, t-ZrO2, and m-ZrO2 is reduced to 1/2, 1/4, and 1/5 of the perfect surface with the assistance of oxygen vacancies. Furthermore, the activation of CO2 on the ZrO2 surface where oxygen vacancies are present, and changes from an endothermic reaction to an exothermic reaction. This finding demonstrates that the presence of oxygen vacancies promotes the activation of CO2 both kinetically and thermodynamically. These results could provide guidance for the high-efficient utilization of CO2 at an atomic scale.
Graphical abstract

识别二维码查看全文



文章中观点仅代表作者个人观点,不代表本网站的观点和看法。
神州学人杂志及神州学人网原创文章转载说明:如需转载,务必注明出处,违者本网将依法追究。